##########
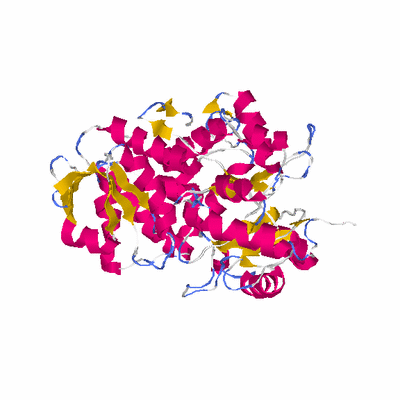
# PROTEIN: Q2246_545_319.5wLII_11247_15 515 319 # Category_C2
# Length: 515
# Weight: 58343.63
# Similarity: UniProt_B6ALJ6, BLAST_B6ALJ6, PDB_templates_INFO
# Best Pcover Model PDB N_AA SISC E-val Seq_ID LAL Overlap
# Cat 77.86 M_03 2hgs_A 474 1 4e-06 13.000 401:469 (25-459:7-441)
# Evl 52.62 M_00 2io8_A 619 10 3e-43 12.000 271:312 (70-355:257-553)
# Sid 65.63 M_00 2io8_A 619 10 4e-40 13.000 338:395 (5-355:172-553)
# Cov 83.69 M_04 2hgs_A 474 1 2e-15 12.000 431:503 (25-490:7-474)
# LaL 23.69 M_03 2qbu_A 232 2 0.062 10.000 122:128 (164-287:61-186)
# OvN 77.28 M_04 1m0w_A 491 4 1e-16 12.000 398:522 (4-466:9-467)
# OvC 82.33 M_01 2hgs_A 474 1 1e-32 12.000 424:491 (35-490:16-474)
PDB: 2hgs PDBsum
HEADER AMINE/CARBOXYLATE LIGASE 04-JAN-99 2HGS
TITLE HUMAN GLUTATHIONE SYNTHETASE
SOURCE 2 ORGANISM_SCIENTIFIC: HOMO SAPIENS;
SOURCE 3 ORGANISM_COMMON: HUMAN;
SOURCE 4 ORGANISM_TAXID: 9606;
COMPND 4 EC: 6.3.2.3;
KEYWDS AMINE/CARBOXYLATE LIGASE
SCOP: c.30 PreATP-grasp domain
SCOP: c.30.1 PreATP-grasp domain
SCOP: c.30.1.4 Eukaryotic glutathione synthetase, substrate-binding domain
SCOP: d.142 ATP-grasp
SCOP: d.142.1 Glutathione synthetase ATP-binding domain-like
SCOP: d.142.1.6 Eukaryotic glutathione synthetase ATP-binding domain
PDB: 2io8 PDBsum
HEADER LIGASE, HYDROLASE 10-OCT-06 2IO8
TITLE E. COLI BIFUNCTIONAL GLUTATHIONYLSPERMIDINE
TITLE 2 SYNTHETASE/AMIDASE INCOMPLEX WITH MG2+ AND ADP
SOURCE 2 ORGANISM_SCIENTIFIC: ESCHERICHIA COLI;
SOURCE 3 ORGANISM_TAXID: 562;
COMPND 7 EC: 6.3.1.8, 3.5.1.78;
KEYWDS BIFUNCTIONAL GLUTATHIONYLSPERMIDINE SYNTHETASE/AMIDASE,
KEYWDS 2 LIGASE, HYDROLASE
SCOP: c.30 PreATP-grasp domain
SCOP: c.30.1 PreATP-grasp domain
SCOP: c.30.1.7 Glutathionylspermidine synthase substrate-binding domain-like
SCOP: d.3 Cysteine proteinases
SCOP: d.3.1 Cysteine proteinases
SCOP: d.3.1.15 CHAP domain
SCOP: d.142 ATP-grasp
SCOP: d.142.1 Glutathione synthetase ATP-binding domain-like
SCOP: d.142.1.8 Glutathionylspermidine synthase ATP-binding domain-like
PDB: 2qbu PDBsum
HEADER TRANSFERASE 18-JUN-07 2QBU
TITLE CRYSTAL STRUCTURE OF METHANOTHERMOBACTER THERMAUTOTROPHICUS
TITLE 2 CBIL
SOURCE 2 ORGANISM_SCIENTIFIC: METHANOTHERMOBACTER
SOURCE 4 ORGANISM_TAXID: 187420;
KEYWDS METHYLTRANSFERASE
SCOP: none
PDB: 1m0w PDBsum
HEADER LIGASE 14-JUN-02 1M0W
TITLE YEAST GLUTATHIONE SYNTHASE BOUND TO GAMMA-GLUTAMYL-CYSTEINE,
TITLE 2 AMP-PNP AND 2 MAGNESIUM IONS
SOURCE 2 ORGANISM_SCIENTIFIC: SACCHAROMYCES CEREVISIAE;
SOURCE 3 ORGANISM_COMMON: BAKER'S YEAST;
SOURCE 4 ORGANISM_TAXID: 4932;
COMPND 5 EC: 6.3.2.3;
KEYWDS AMINE/CARBOXYLATE LIGASE, STRUCTURAL GENOMICS, PSI, PROTEIN
KEYWDS 2 STRUCTURE INITIATIVE, NEW YORK SGX RESEARCH CENTER FOR
KEYWDS 3 STRUCTURAL GENOMICS, NYSGXRC
SCOP: c.30 PreATP-grasp domain
SCOP: c.30.1 PreATP-grasp domain
SCOP: c.30.1.4 Eukaryotic glutathione synthetase, substrate-binding domain
SCOP: d.142 ATP-grasp
SCOP: d.142.1 Glutathione synthetase ATP-binding domain-like
SCOP: d.142.1.6 Eukaryotic glutathione synthetase ATP-binding domain